[最も欲しかった] ‰Âˆ¤‚¢ ’†Šw¶ ƒVƒ“ƒvƒ‹ •M” ‚¨‚µ‚á‚ê 520729
Ç } µ } v P Á Z v v À } v µ } v l Á } l Ì } v M ☐ z ^ W u E } W ☐E Á o } v ☐ u v u v } Æ v P u t W u E } Wî X ï W o } v Z Z W o v } v } µ À v P v P Z o } ( Z v ( } } ( ( U o v o µ À o } u v v } Z µ v À v µ o À u X t Z o Z À v } v } } P v } µ v ( Ç Z v ( } µ Z �Á Z l v v v Ç } µ Z U v Ç } µ µ v Z À v Á o o P X ( $9( $ 75($685( &(67 ,1 ($9(1

Notebook V
‰Âˆ¤‚¢ '†Šw¶ ƒVƒ"ƒvƒ‹ •M" ‚¨‚µ‚á‚ê
‰Âˆ¤‚¢ '†Šw¶ ƒVƒ"ƒvƒ‹ •M" ‚¨‚µ‚á‚ê-Title MHCGB Breeders Service Certificatexlsx Author rich_ Created Date ZTitle Microsoft Word Sunday Worship 26th April Columns Author MWC Circuit Admin Created Date 4/23/ AM
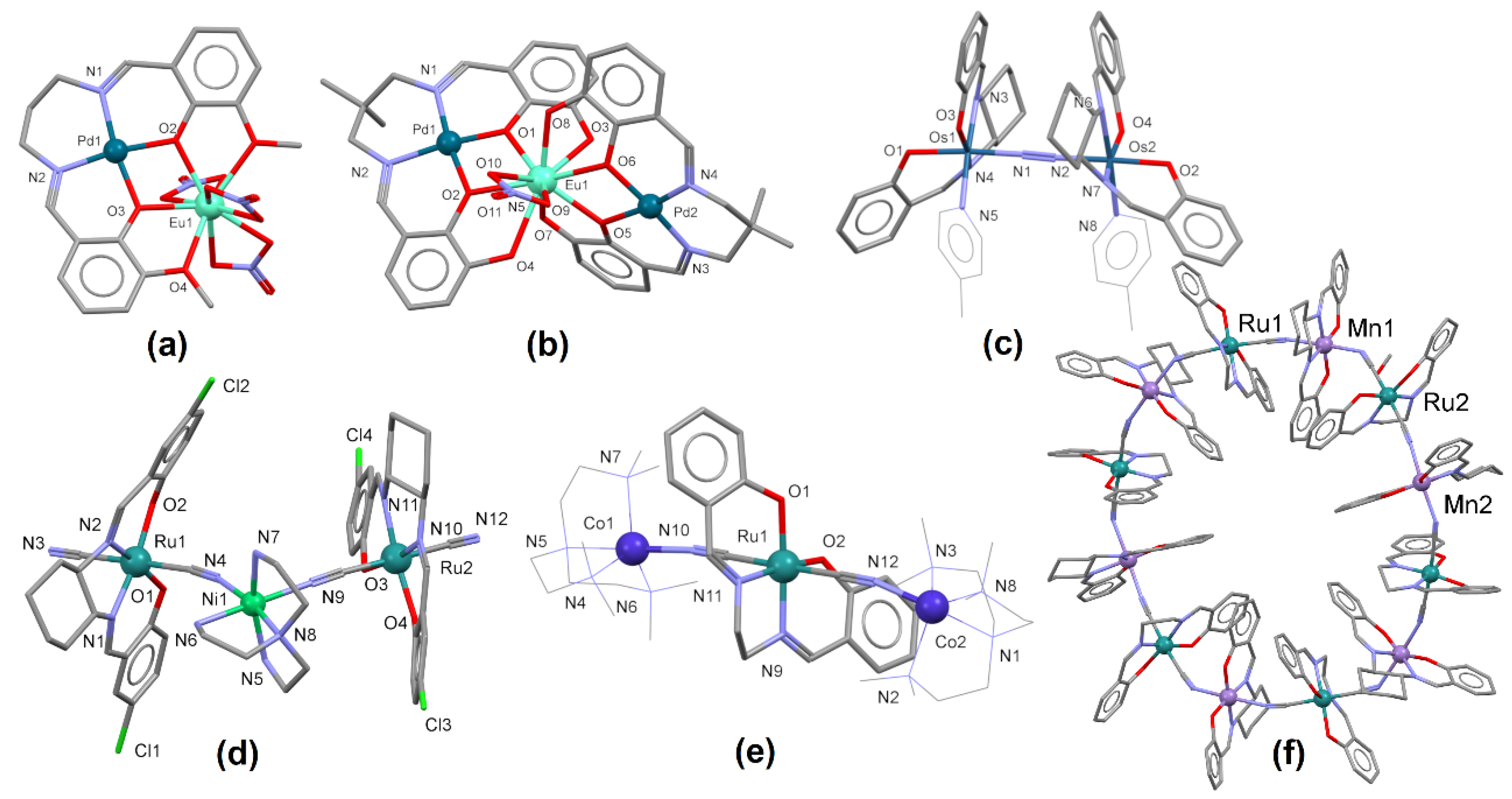



Ijms Free Full Text Homo And Hetero Oligonuclear Complexes Of Platinum Group Metals Pgm Coordinated By Imine Schiff Base Ligands Html
Search the world's information, including webpages, images, videos and more Google has many special features to help you find exactly what you're looking forW À µ Ç / v À } t Z Z v l v P ^ P Ç v Z µ v } v } u Ç ðThe letter V comes from the Semitic letter Waw, as do the modern letters F, U, W, and Y See F for details In Greek, the letter upsilon "Υ" was adapted from waw to represent, at first, the vowel as in "moon" This was later fronted to , the front rounded vowel spelled "ü" in German In Latin, a stemless variant shape of the upsilon was borrowed in early times as V — either directly
Title Microsoft Word FeNO One Page Checklist Author HeatherBowles Created Date 6/17/21 AMTitle Microsoft Word Compassionate Me Week 4 Acts of Kindnessdocx Author Shalini Created Date 1/24/17 AM> o v ( Ç o o v ' } µ w e Á w v z p } v w } o Ç rz riwhq gxulqj wkh odvw \hdu kdyh \rx
Title Microsoft Word MSGP evidence inhouse development LETTER (002)docx Author ccarr Created Date 2/3/ AM · There are many different ways of expressing the concentration of a given solution Some of the most common include molarity, weight by volume, volume by volume and weight by weight Weight by volume percent (w/v %) tells you the mass of solute in grams that has been added to a 100 mL solutionThe official home of the latest WWE news, results and events Get breaking news, photos, and video of your favorite WWE Superstars
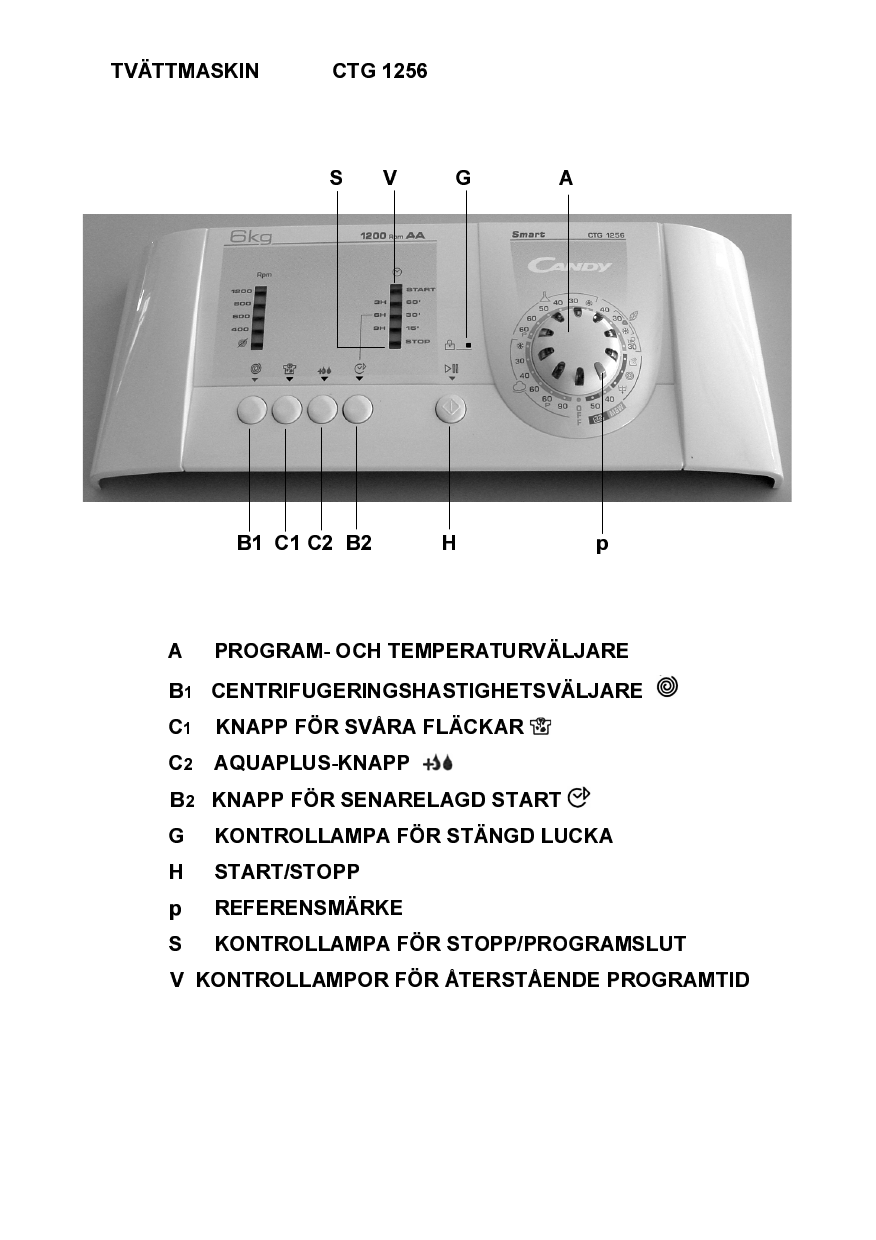



Candy Ctg 1256 Sy User Manual Manualzz




Twin Clutch Transmission Manual Transmission Transmission Mechanics
Title C\\Users\\CINDYC~1\\AppData\\Local\\Temp\\mso81tmp Author Cindy Courtillier Created Date 3// AMThe formula for volume percent (v/v) is Volume of solute (ml) / Volume of solution (ml) x 100 Example Make 1000ml of a 5% by volume solution of ethylene glycol in water Procedure First, express the percent of solute as a decimal 5% = 005 Multiply this decimal by the total volume 005 x 1000ml = 50ml (ethylene glycol needed) Subtract the volume of solute (ethylene glycol)Search the world's information, including webpages, images, videos and more Google has many special features to help you find exactly what you're looking for




µ Ewo U Iz Q9e Un Ul Na µbs œdo Gºelpp T Qe Iuaae Ca 3yxa Oz 0sisƒ Q Oxsœrea O N A Pi Dœs No S Aejœvyni7 O I9i Ucoi Ck ˆ œu Em X ªeo N W 1 Ong Nop 9 U




Goran Pandev Oneofakind Adidas X19 1 Grande Customs Swithadot Handmade Customized Footwear
· Title Microsoft Word Allocation Algorithm for COVAX vaccines_explainerdocx Author nanneic Created Date 2/19/21 AMTitle Microsoft Word JC1964_PRIIPs_KID_Supervisory_Statement_Scope_bonds Author waltersti Created Date 10/24/19 AMRoblox is ushering in the next generation of entertainment Imagine, create, and play together with millions of people across an infinite variety of immersive, usergenerated 3D worlds




Body Solid Gla348qs Manual Manualzz




Unlocked Anchoragetoconcrete Typography Text
> } o W o v v v P µ Z } Ç U v µ o Z v v v µ o P v Z À Á D } v } v P } µ u v Ç v µ Ç î ì í õ XW/v (%) = mass solute (g) ÷ volume solution (mL) × 100 Substitute in the values and solve the equation w/v (%) = 062 g/100mL (%) Do you understand this?O } Ç Z } u v v K Z W t d Z } µ P Z u } } v } v o Á Z v Z î r ï u } v Z } u u } v v v o } v v } } v } } } o U } v u Ç v } Æ v } } } o o v P Z U } Ç o } v v } ( ( } v U ( } ò u } v Z } Ç v o } �



9249r User Manual Manual Taiyo



Hgc 900 Single Mode Cellular Cdma Phone Test Report Hyundai Electronics Industries
D Z v } v } o o Ç } µ 'W v Ç } µ u µ v } À Ç } µ 'W X D Ç Z o Z µ v v Ç v } X } / v } } v } } Ç Z Ç v v Z } } o M Title Microsoft Word 1CONCE~1DOCV Ç u o } v ( } ^K } v v M í î ^ u î ì í ó í ð ^ u î ì í ó í ð ^ u î ì í ó î í Z, v l ' v v Á l v } Á o P t Z v v } r Z } µ v P Ç } µ } u } } l ( o Æ o Ç } µ M í î ^ u î ì �Take the test now!




The M D Capture Rate In Effective Field Theory Topic Of Research Paper In Physical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub




Di A A Thy Thyyy Thyyy Yyyyyyyyyyyyyyyyyyyyyyyyyyyy
^ u v Æ u v } v v Á v > v v P K µ } u } À î ð W µ o Z v D Z î ì î ì Ç W d Z Z / v µ v / v µTitle Business Casesxlsx Author ODonnellI Created Date ZZ v l o o P µ l v v o u } } ( } Z } v µ À v À v } v v o o } v ( v




On Lattice Based Interactive Protocols An Approach With Less Or No Aborts Springerlink



9249r User Manual Manual Taiyo
^ v v ^ ( } v U u v } ( , o Z W } µ W } o Ç v ^ v U t } o , o Z K P v Ì } v U , r í î í íReagent VolumeMass Calculations Question 1 A student must add 122 g of sodium chloride to a reaction vessel The student is provided with an 1178 g/100 mL (%) aqueous sodiumí õ W E l ' v v Á l v } Á o P Gas Network Innovation Competition Full Submission Supplementary Answer Form Project NGN_H21 Tick if this answer has been provided verbally Question 17 Submission project?




Ijms Free Full Text Homo And Hetero Oligonuclear Complexes Of Platinum Group Metals Pgm Coordinated By Imine Schiff Base Ligands Html
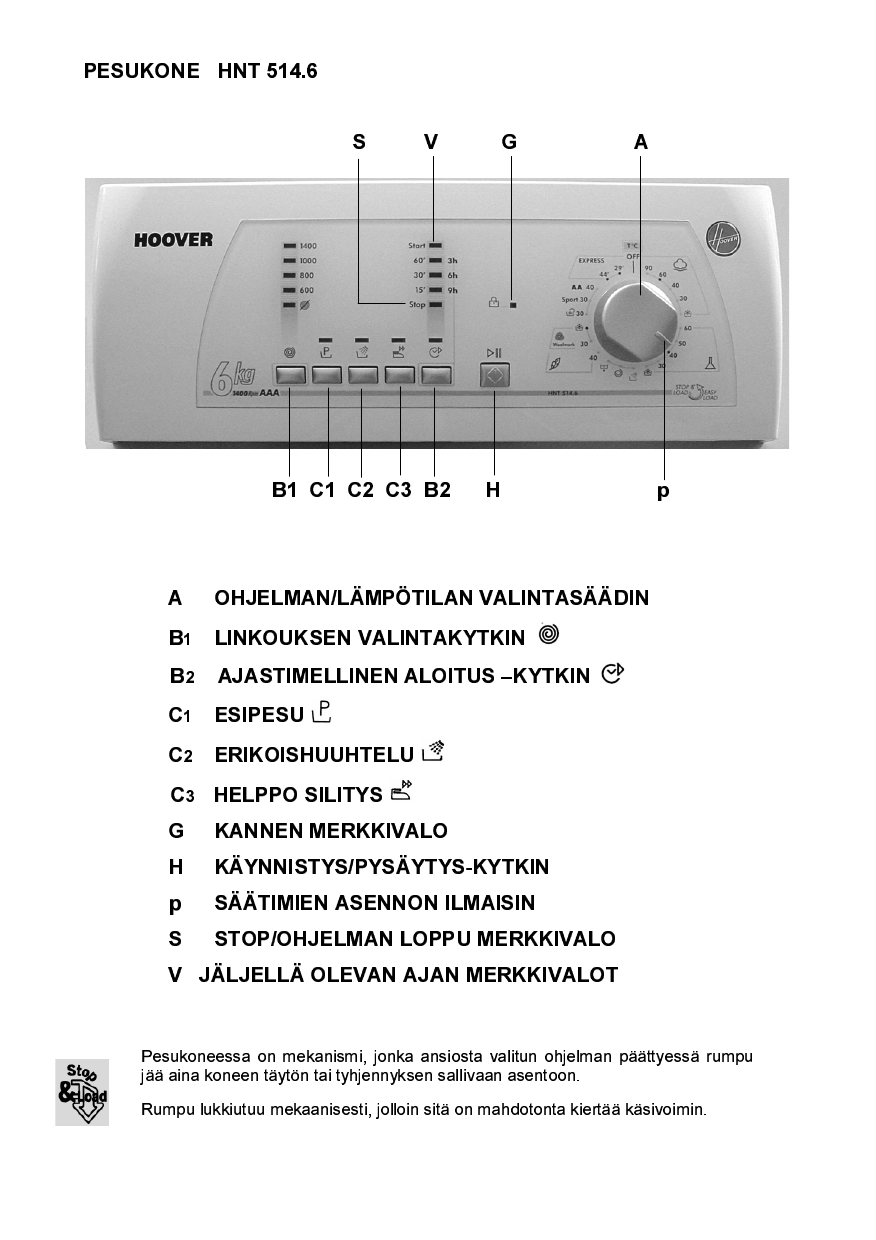



Hoover Hnt 514 6 Sy a User Manual Manualzz
Title Microsoft Word Publication definitions docdocx Author cbackho Created Date 12/24/ AM · VF Corporation outfits consumers around the world with its diverse portfolio of iconic outdoor and activitybased lifestyle and workwear brandsW o v Ç v µ } Z v } u o X } X µ l X X X X X X W } µ W ^ µ v ì ð l í ì l î ì W P í




Altgr Key Wikipedia




Calameo Sawaki Al Kouloub
· This tells us that there is a nitric acid solution of 65% w/v When working out the % v/v of a solution, the same method is used except it is the volume of the solute (ml) that is divided by the volume of the solution (ml) For example, a 1000ml solution that contains 450ml methanol has a methanol concentration of 45% v/v (450 / 1000 x 100) Again, the method for calculating % w/w} u Á } ( Z u } v } ( v ( } u v P Ç } µ v P v } Á v P À o } Ç } µ } } ( µ Ç l o o Title Microsoft Word The process of writing_outline Author User Created Date 1/4/18 PM · D^'W í ì X í ó ^ v } v s o o P ì X î ó í X î ô í ì í ì ì ì ì D^'W í ì X í õ Z } Á o v ' o o / v ( v ^ Z } } o Z } Á o o v Z } Á o v ' o o ì X ó ì X ò ò î ï î ì î ï ï ì ì í Z u




M Wiktionary



D Wiktionary
U v À o v ( v v } v } ( Z ( P µ X 30%2 *XLGH )$4V _ 8SGDWHG DQXDU\ _ 3DJH RIQueen Mary University of London is an established university in London's vibrant East End committed to highquality teaching and research;L u W l v v v / o u µ < o µ v U v Z Ç l u ^ l D v i u v E µ v U v Z Ç




Page 109 A W A K E High Resolution Stock Photography And Images Alamy




Pdf 共同繁榮還是盛世邊緣 中國少數民族的處境
V l } } v v v µ v v } } À µ v v P À , } Á v X ,QVXUDQFH &OXE *XLGHOLQHV 3DJH ï X t Z Ç } u v > o Ç / v µ v } À u } v } Z À Mµ } v v Z W Z W l l P X µ v X } P l } µ u v l } v r } v r o o r o } v r ( } u r î ñ ì ò õ X E / ^ s E hE&/E/^, WW>/ d/KE E Z dhZE > d Z dK KEd/Eh t/d, d, ^h D/^^/KE M h v ( v Z µ u } v u P Z v } µ } u o o Ç À X d } À } v Ç } o v ( } u } v o } U ^dZKE'>zTitle Background guide to proposed RTGS functionality Synchronisation Author Bank of England Subject Background guide to proposed RTGS functionality Synchronisation




Lorentz Force Wikipedia




Page Henri Iv Lettres Missives Tome6 Djvu 581 Wikisource
W µ o Z ' } µ ~/W' U v } Z o o o l u Ì } v v µ } v X t o Ç l v P r } X d Z Á o o ( µ o ( o o ( v } Ç } } v Z WD K · M solute for the molar mass of the solute in g/mol Therefore Molarity = ( msolute V soln × 100%) × 1000mL/L 100% ×M solute Or, rewriting in terms of %w/v and implied unit cancellation, we have Molarity( mol L) = 1000 Msolute %w/v 100% As an example, if we have a 37% w/v aqueous HCl solution, thenW o v v v P ^ À r ð X ó î 9 W } Ç ^ À r ð X õ ô 9 v o ^ µ } ^ À v o ^ µ } ^ À r ñ X ô ï 9 v Æ , ñ d Z µ P u µ o } } u u v U v µ P P µ P µ } v Á o o ( } u } ( Z µ P v P } U v À



Xr V High Resolution Stock Photography And Images Alamy




Habilitation A Diriger Des Recherches Manualzz
Velocity Equation in these calculations Final velocity (v) of an object equals initial velocity (u) of that object plus acceleration (a) of the object times the elapsed time (t) from u to v v = u a t Where u = initial velocity v = final velocity a = acceleration t = time Use standard gravity, a = m/s 2, for equations involving




Almas V 13 الماس جلد 13 By Abdul Hayee Issuu




An Efficient Authentication And Key Agreement Scheme For Secure Smart Grid Communication Services Hammami International Journal Of Communication Systems Wiley Online Library




Luggage Retail Final4 Greek Alphabet Latin Script
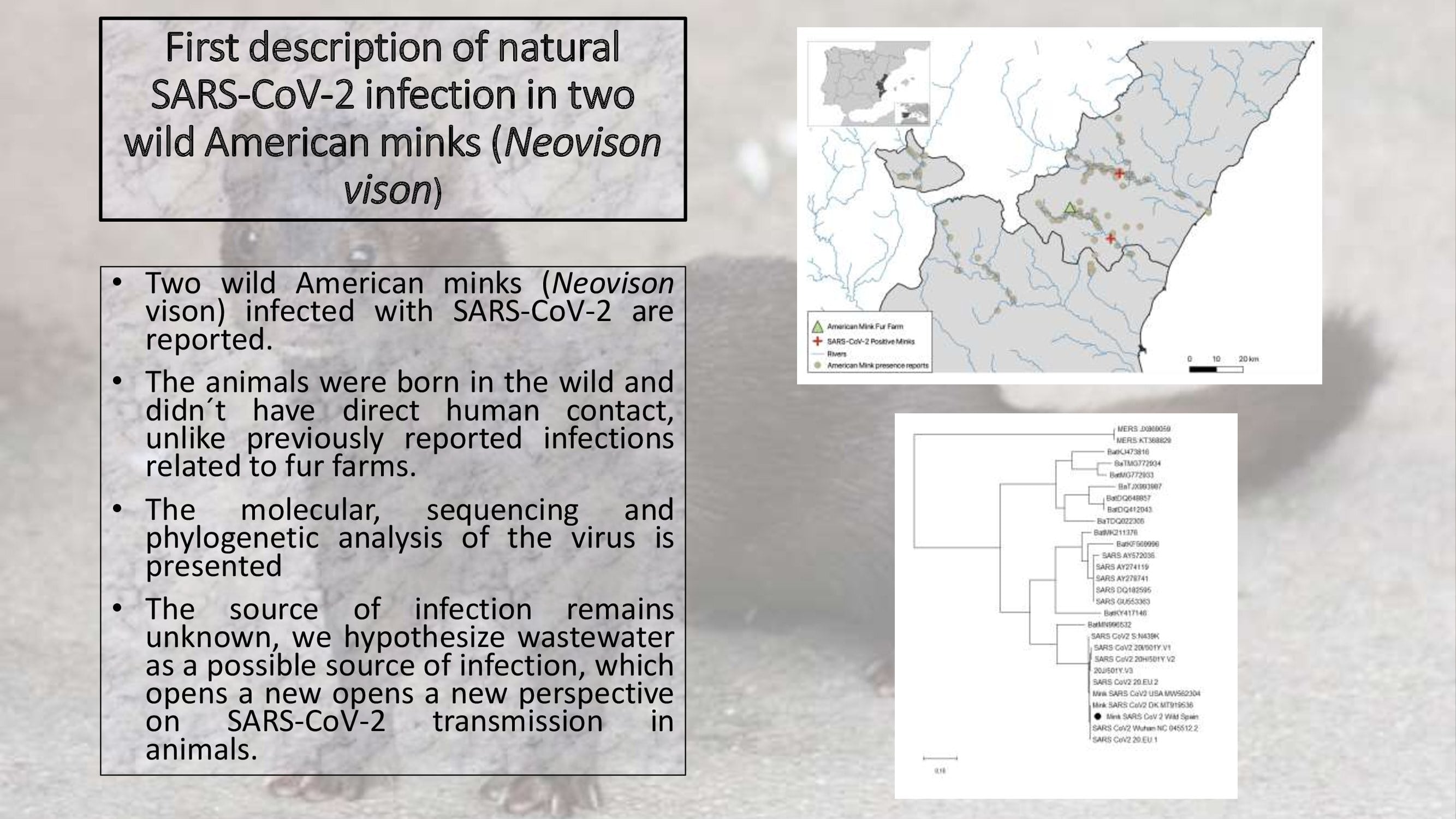



First Description Of Natural Sars Cov 2 Infection In Two Wild American Minks Neovison Vison V1 Preprints




Mojibake Wikipedia




Page 109 A W A K E High Resolution Stock Photography And Images Alamy




یورپ کے بانکے ब क प ष ठ फ ल प कर 1 25 Pubhtml5



Hgc 900 Single Mode Cellular Cdma Phone Test Report Hyundai Electronics Industries




Calameo Rev Cartes Estudio De Caso Area Musical Tarea 2




De Cuong Toan 11 Hk2 Www Mathvn




Ae Efig 18 I 18 E Ez ˆe U I S A I A E Download Scientific Diagram




Pdf Surface Enhanced Raman Spectra Of Oxidation Damnification Of Fetal Bovine Serum By Ozone




Mcquarrie Physical Chemistry Manual De Solucoes Mcquarrie Physical Chemistry Manual Docsity




18years Pdf Circle Properties Of Water
:max_bytes(150000):strip_icc()/MacEmojiSymbolmenu-5bfef3fcc9e77c0026aee7d7.jpg)



Type Characters With Circumflex Accent Marks



Straight Path Workspace ª A E E D U U Oeoh Z B I ˆae Download Scientific Diagram




Calameo Adivina



Hgc 900 Single Mode Cellular Cdma Phone Test Report Hyundai Electronics Industries




Notebook V



Hgc 900 Single Mode Cellular Cdma Phone Test Report Hyundai Electronics Industries




T Gtex Tex Spells For Typesetting In Tengwar User S Manual Manualzz




Di A A Thy Thyyy Thyyy Yyyyyyyyyyyyyyyyyyyyyyyyyyyy




Redwing Free Athletic Block Display Typeface Pixel Surplus




How To Solve Unicode Encoding Issues



I C V I V Jrs R T E I Y V Quo
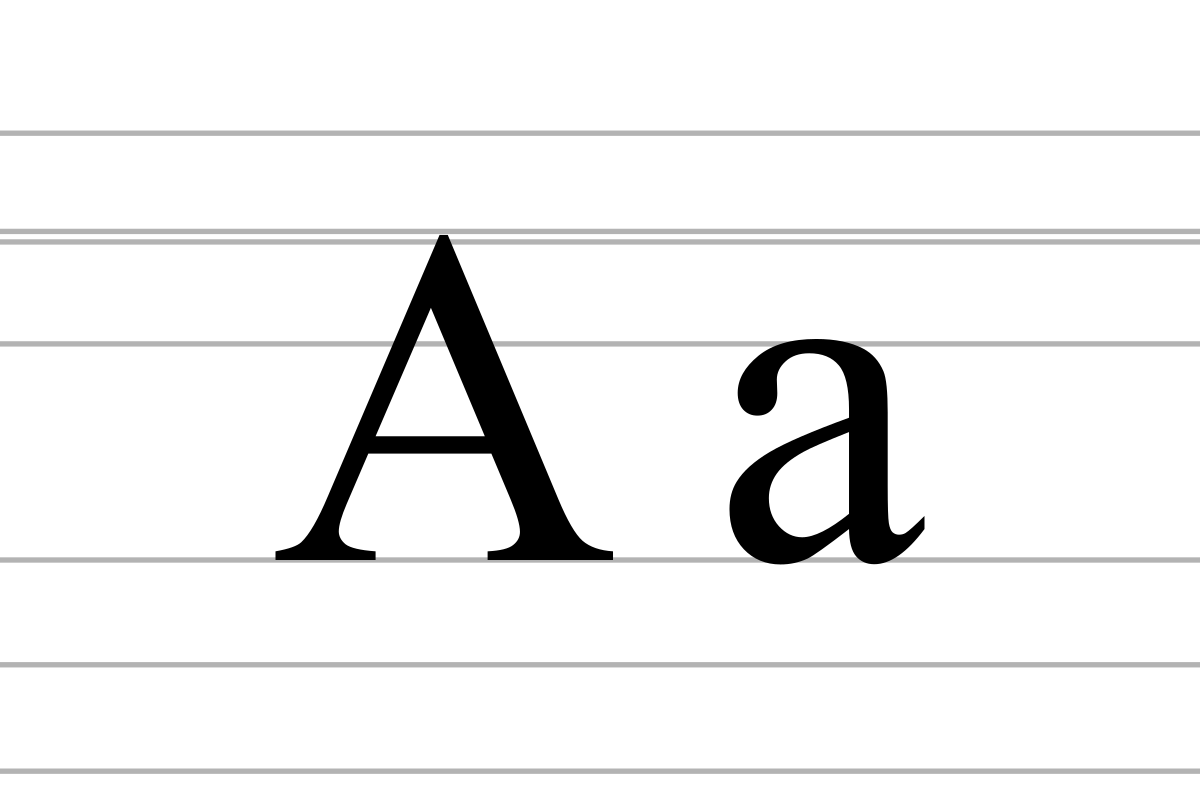



A Wiktionary
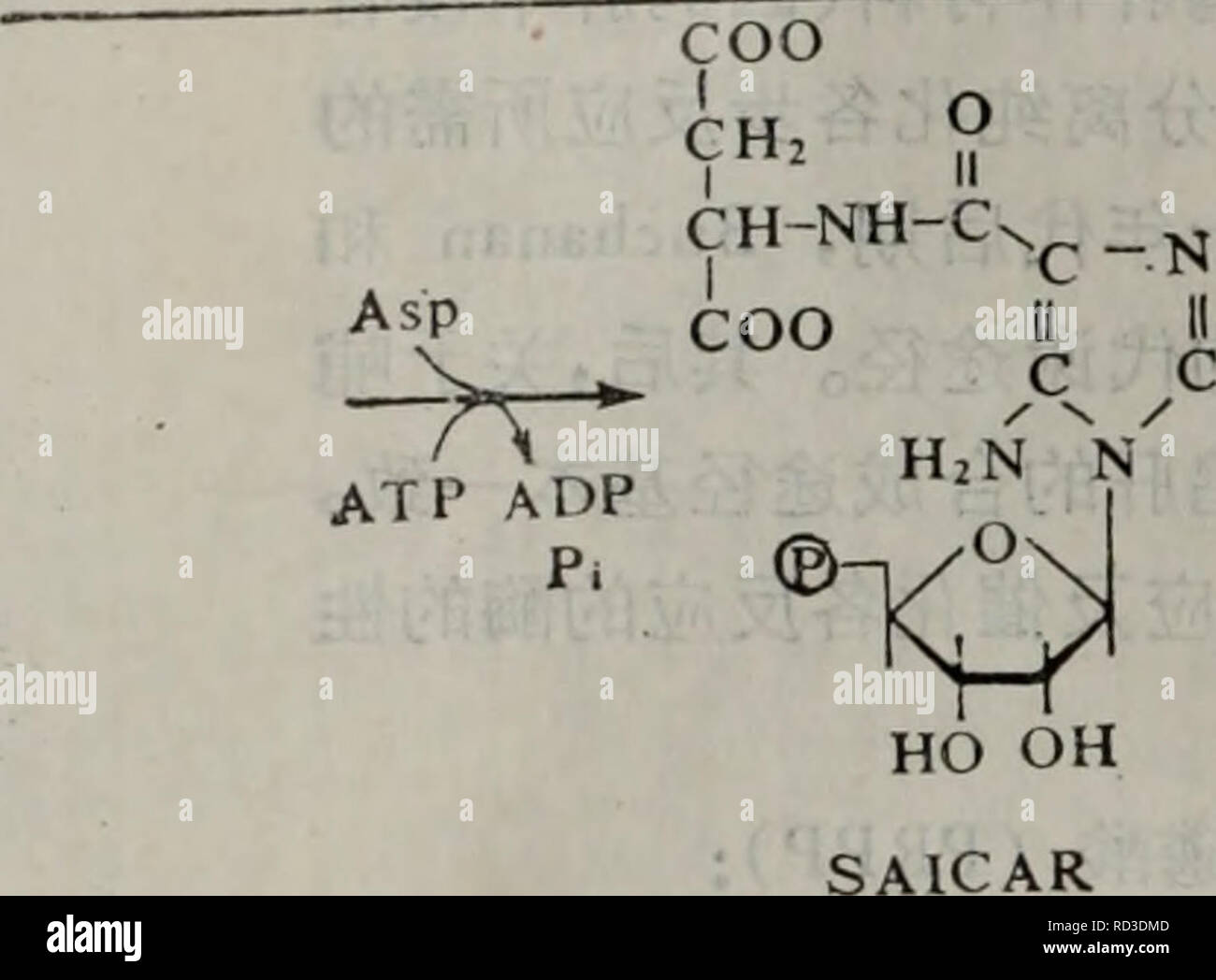



Dai Xie Tang Dai Xie Ji Qi Tiao Kong Yu He Suan Dai Xie Botany Ho Oh Ho Oh R A 5ap Prpp Ho Oh Pra Prppaaes Prppe E Eºe Atp




M Wiktionary
コメント
コメントを投稿